Photosynthesis Defined
A great variety of living things on earth, including all green plants, synthesize their foods from simple molecules, such as carbon dioxide and water. For this process, the organisms require energy, and that energy is derived from sunlight.
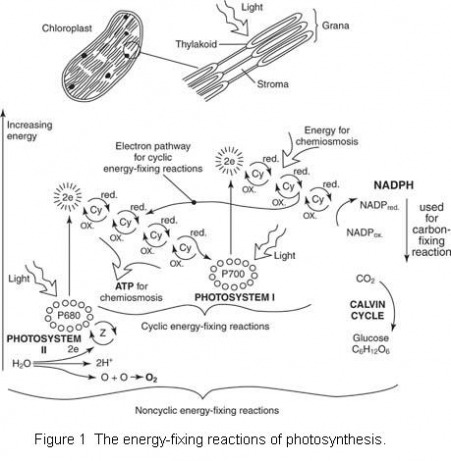
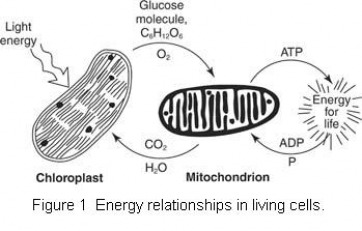
Figure 1 shows the energy relationships in living cells. Light energy is captured in the chloroplast of plant cells and used to synthesize glucose molecules, shown as C6H12O6. In the process, oxygen (O2) is released as a waste product. The glucose and oxygen are then used in the mitochondrion of the plant and animal cell, and the energy is released and used to fuel the synthesis of ATP from ADP and P. In the reaction, C02 and water are released in the mitochondrion to be reused in photosynthesis in the chloroplast.
The process of utilizing energy to synthesize carbohydrate molecules is referred to as photosynthesis. Photosynthesis is actually two separate processes. In the first process, energy-rich electrons flow through a series of coenzymes and other molecules. This electron energy is trapped. During the trapping process, adenosine triphosphate (ATP) molecules and molecules of nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) are formed. Both ATP and NADPH are rich in energy. These molecules are used in the second half of the process, where carbon dioxide molecules are bound into carbohydrates to form organic substances such as glucose.
Chloroplast
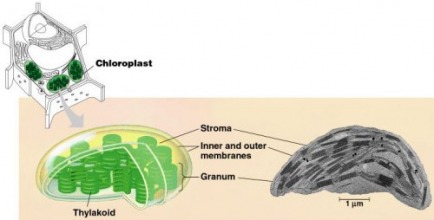
The organelle in which photosynthesis occurs (in the leaves and green stems of plants) is called the chloroplast. Chloroplasts are relatively large organelles, containing a watery, protein-rich fluid called stroma. The stroma contains many small structures composed of membranes that resemble stacks of coins. Each stack is a granum (the plural form is grana). Each membrane in the stack is a thylakoid. Within the thylakoid membranes (or thylakoids) of the granum, many of the reactions of photosynthesis take place. The thylakoids are somewhat similar to the cristae of mitochondria.
Photosystems
Pigment molecules organized into photosystems capture sunlight in the chloroplast. Photosystems are clusters of light-absorbing pigments with some associated molecules—proton (hydrogen ion) pumps, enzymes, coenzymes, and cytochromes. Each photosystem contains about 200 molecules of a green pigment called chlorophyll and about 50 molecules of another family of pigments called carotenoids. In the reaction center of the photosystem, the energy of sunlight is converted to chemical energy. The center is sometimes called a light-harvesting antenna.
There are two photosystems within the thylakoid membranes, designated photosystem I and photosystem II. The reaction centers of these photosystems are P700 and P680, respectively. The energy captured in these reaction centers drives chemiosmosis, and the energy of chemiosmosis stimulates ATP production in the chloroplasts.
Process of Photosynthesis
The process of photosynthesis is conveniently divided into two parts: the energy-fixing reaction (also called the light reaction) and the carbon-fixing reaction (also called the light-independent reaction, or the dark reaction).
Energy-fixing reaction
The energy-fixing reaction of photosynthesis begins when light is absorbed in photosystem II in the thylakoid membranes. The energy of the sunlight, captured in the P680 reaction center, activates electrons to jump out of the chlorophyll molecules in the reaction center. These electrons pass through a series of cytochromes in the nearby electron-transport system.
After passing through the electron transport system, the energy-rich electrons eventually enter photosystem 1. Some of the energy of the electron is lost as the electron moves along the chain of acceptors, but a portion of the energy pumps protons across the thylakoid membrane, and this pumping sets up the potential for chemiosmosis.
The spent electrons from P680 enter the P700 reaction center in photosystem I. Sunlight now activates the electrons, which receive a second boost out of the chlorophyll molecules. There they reach a high energy level. Now the electrons progress through a second electron transport system, but this time there is no proton pumping. Rather, the energy reduces NADP. This reduction occurs as two electrons join NADP and energize the molecule. Because NADP acquires two negatively charged electrons, it attracts two positively charged protons to balance the charges. Consequently, the NADP molecule is reduced to NADPH, a molecule that contains much energy.
Because electrons have flowed out of the P680 reaction center, the chlorophyll molecules are left without a certain number of electrons. Electrons secured from water molecules replace these electrons. Each split water molecule releases two electrons that enter the chlorophyll molecules to replace those lost. The split water molecules also release two protons that enter the cytoplasm near the thylakoid and are available to increase the chemiosmotic gradient.
The third product of the disrupted water molecules is oxygen. Two oxygen atoms combine with one another to form molecular oxygen, which is given off as the byproduct of photosynthesis; it fills the atmosphere and is used by all oxygen-breathing organisms, including plant and animal cells.
What has been described above are the noncyclic energy-fixing reactions (see Figure 1 ). Certain plants are also known to participate in cyclic energy-fixing reactions. These reactions involve only photosystem I and the P700 reaction center. Excited electrons leave the reaction center, pass through coenzymes of the electron transport system, and then follow a special pathway back to P700. Each electron powers the proton pump and encourages the transport of a proton across the thylakoid membrane. This process enriches the proton gradient and eventually leads to the generation of ATP.
ATP production in the energy-fixing reactions of photosynthesis occurs by the process of chemiosmosis. Essentially, this process consists of a rush of protons across a membrane (the thylakoid membrane, in this case), accompanied by the synthesis of ATP molecules. Biochemists have calculated that the proton concentration on one side of the thylakoid is 10,000 times that on the opposite side of the membrane.
In photosynthesis, the protons pass back across the membranes through channels lying alongside sites where enzymes are located. As the protons pass through the channels, the energy of the protons is released to form high-energy ATP bonds. ATP is formed in the energy-fixing reactions along with the NADPH formed in the main reactions. Both ATP and NADPH provide the energy necessary for the synthesis of carbohydrates that occurs in the second major set of events in photosynthesis.
Carbon-fixing reaction
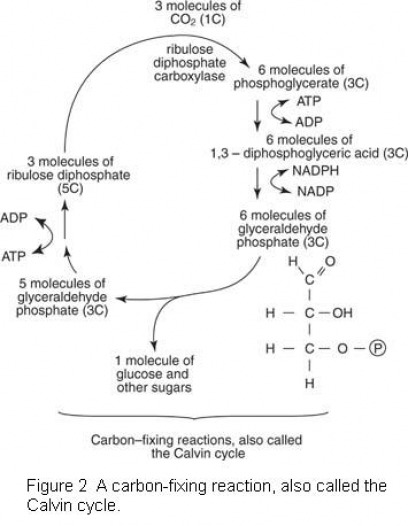
Glucose and other carbohydrates are synthesized in the carbon-fixing reaction of photosynthesis, often called the Calvin cycle for Melvin Calvin, who performed much of the biochemical research (see Figure 2 ). This phase of photosynthesis occurs in the stroma of the plant cell.
In the carbon-fixing reaction, an essential material is carbon dioxide, which is obtained from the atmosphere. The carbon dioxide is attached to a five-carbon compound called ribulose diphosphate. Ribulose diphosphate carboxylase catalyzes this reaction.
After carbon dioxide has been joined to ribulose diphosphate, a six-carbon product forms, which immediately breaks into two three-carbon molecules called phosphoglycerate. Each phosphoglycerate molecule converts to another organic compound, but only in the presence of ATP. The ATP used is the ATP synthesized in the energy-fixing reaction. The organic compound formed converts to still another organic compound using the energy present in NADPH. Again, the energy-fixing reaction provides the essential energy. The organic compounds that result each consist of three carbon atoms. Eventually, the compounds interact with one another and join to form a single molecule of six-carbon glucose. This process also generates additional molecules of ribulose diphosphate to participate in further carbon-fixing reactions.
Glucose can be stored in plants in several ways. In some plants, the glucose molecules are joined to one another to form starch molecules. Potato plants, for example, store starch in tubers (underground stems). In some plants, glucose converts to fructose (fruit sugar), and the energy is stored in this form. In still other plants, fructose combines with glucose to form sucrose, commonly known as table sugar. The energy is stored in carbohydrates in this form. Plant cells obtain energy for their activities from these molecules. Animals use the same forms of glucose by consuming plants and delivering the molecules to their cells.
All living things on earth depend in some way on photosynthesis. It is the main mechanism for bringing the energy of sunlight into living systems and making that energy available for the chemical reactions taking place in cells.
Photosynthesis Formula
6CO2 + 6 H2O + sunlight --> 6O2 + C6H12O6
Carbon Dioxide + Water + Sunlight --> Oxygen + Glucose